FAQs about Ultomiris
There is no known interaction between alcohol and Ultomiris. However, because alcohol can affect some treatments, patients should talk with their healthcare team before drinking alcohol while on Ultomiris.
There is no information on the use of Ultomiris during pregnancy in humans, however, in animal studies, a similar medication caused harm to the developing fetus. That risk must be weighed with the fact that pregnancy in women with untreated paroxysmal nocturnal hemoglobinuria carries a higher risk of complications for both mother and baby. Patients who are pregnant or planning to become pregnant should talk with their healthcare team.
Hair loss and weight gain have not been reported as side effects of Ultomiris in people with paroxysmal nocturnal hemoglobinuria. Patients should talk with their healthcare team if they experience any unexpected side effects.
In clinical trials, some people with paroxysmal nocturnal hemoglobinuria who were treated with Ultomiris started to see reductions in biomarkers of red blood cell destruction (hemolysis) within two weeks. However, results may vary, so patients should talk with their healthcare team about how Ultomiris may help in their particular case.
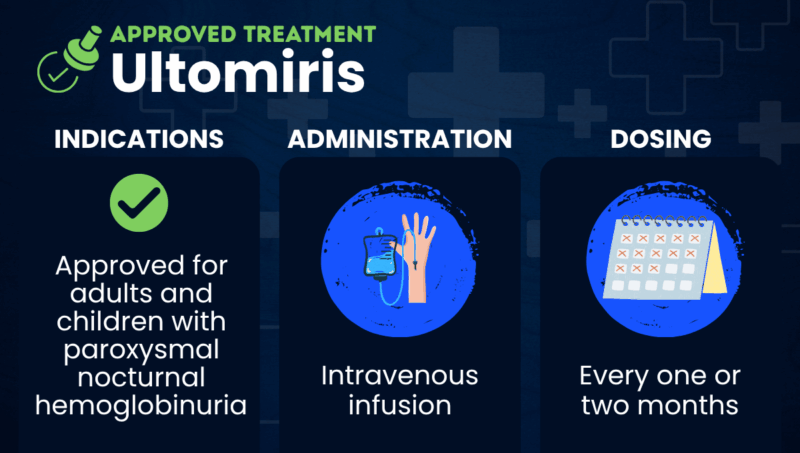
The U.S. Food and Drug Administration approved Ultomiris for adults with paroxysmal nocturnal hemoglobinuria in 2018 and for children, ages 1 month and older, in 2021. Ultomiris is also approved in the U.S. for atypical hemolytic uremic syndrome, generalized myasthenia gravis, and neuromyelitis optica spectrum disorder.
 Fact-checked by
Fact-checked by