FAQs about Soliris
There is no known interaction between alcohol and Soliris. However, because alcohol can interfere with some medications and disease symptoms, it is recommended that patients talk about this issue with their healthcare providers.
The use of Soliris in pregnant women is not well-studied; however, a similar compound (an anti-C5 antibody) tested in animal models showed it could cause harm to a developing fetus. Pregnancy in women with untreated PNH is related to an increased risk of complications for both mother and fetus. Women who are pregnant or are planning to become pregnant should discuss this issue with their healthcare team.
Neither hair loss nor weight gain has been reported in clinical trials as side effects of Soliris treatment in patients with paroxysmal nocturnal hemoglobinuria. Patients are advised to speak with their healthcare provider if they experience any unusual symptoms while on the medication.
Patients with paroxysmal nocturnal hemoglobinuria may see results soon after starting treatment: The TRIUMPH trial showed that Soliris could reduce intravascular hemolysis in a week, and patients treated with the therapy reported improved health-related quality of life after 3 weeks. A significant reduction in hemolysis, which resulted in improvements in anemia and a reduced need for red blood cells transfusions, was evident with Soliris at six months after treatment start. However, each patient is unique and may have different disease manifestations and different responses to treatment. Therefore, a discussion with their healthcare team can help PNH patients understand how Soliris may help in their particular case.
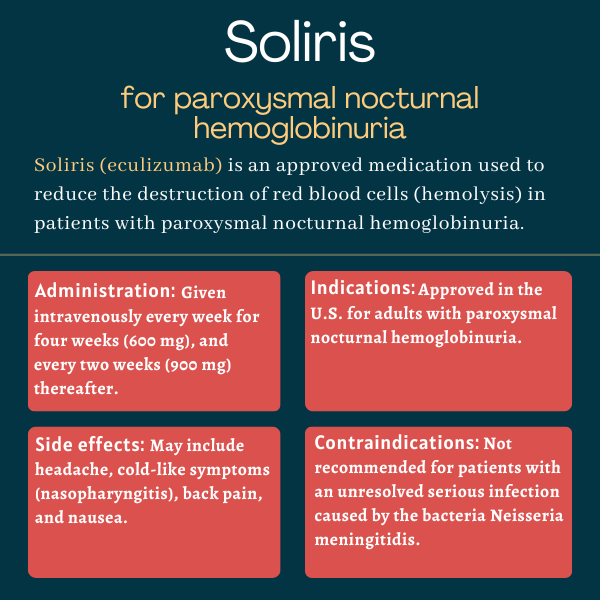
Soliris was approved by the U.S. Food and Drug Administration (FDA) in March 2007 for the treatment of paroxysmal nocturnal hemoglobinuria (PNH). The therapy also is approved in the U.S. for atypical hemolytic uremic syndrome, myasthenia gravis, and neuromyelitis optica spectrum disorder.
Related Articles

 Fact-checked by
Fact-checked by