‘Why Me’ to ‘What’s Next:’ Garrett’s Journey With PNH
Written by |

Please note that Garrett is an adult living with PNH and taking FABHALTA®, compensated for his time by Novartis. Individual results may vary.
FABHALTA (iptacopan) is a prescription medicine used to treat adults with paroxysmal nocturnal hemoglobinuria (PNH). It is not known if FABHALTA is safe and effective in children.
Important Safety Information
What is the most important information I should know about FABHALTA?
FABHALTA is a medicine that affects part of your immune system and may lower your ability to fight infections.
- FABHALTA increases your chance of getting serious infections caused by encapsulated bacteria, including Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b. These serious infections may quickly become life-threatening or fatal if not recognized and treated early.
Please see full Important Safety Information and full Prescribing Information, including Boxed WARNING and Medication Guide.
When Common Symptoms Signal Something More
Growing up in middle Tennessee and now living near a beautiful park outside of Nashville, Garrett had always enjoyed outdoor activities, including hiking with his partner Kait, kayaking and keeping up with his passion for nature photography. But in 2022, a wave of unexplained symptoms hit him hard which made even the most routine parts of his day feel out-of-reach.
At first, Garrett thought that these changes meant that he was out of shape and that going to the gym was the solution, but over time he grew more concerned. Garrett turned to his primary care physician and later a hematologist. After he received a diagnosis of paroxysmal nocturnal hemoglobinuria (PNH), his first thought was, “why me?”
He reflects, “by the time I got the diagnosis, every task you can think of had become harder, and it not only affected me, but also my family.” Garrett decided to commit to working with his care team and not let this rare disease dictate how he was going to live his life.
Finding A Treatment That Fits
Reflecting on his initial treatment plan, Garrett remembers, “I noticed my hemoglobin levels weren’t reaching the levels that I needed to reach.”
In early 2024, Garrett and his doctor developed a treatment plan that was appropriate for him. Together, they decided on switching to FABHALTA® (iptacopan), the first and only oral treatment for adults with PNH taken without infusions or injections.
Garrett recalls, “my doctor was highly recommending it, because I had low hemoglobin levels for so long and I wanted to see improvement.”
PNH occurs when a part of the immune system called the complement system becomes overactive, leading to the destruction of red blood cells, a process known as hemolysis. Until recently, most treatments for PNH involved therapies known as C5 inhibitors. While these treatments represented progress for patients, there remained unmet needs as some patients may still feel some signs and symptoms of PNH due to low hemoglobin (Hb) levels, and most C5 therapies require infusions.1-3
FABHALTA works by inhibiting a protein called Factor B. Unlike C5 inhibitors, which control intravascular hemolysis (IVH), blocking Factor B helps control both types of hemolysis that occur in some patients with PNH: intravascular hemolysis, which happens in the blood vessels; and extravascular hemolysis, which happens most commonly in the liver and spleen.1,2
For Garrett, the shift to an oral treatment was more than just a medical change. “When I first started taking FABHALTA, my doctor told me that my hemoglobin was rising. Prior to FABHALTA, my hemoglobin level usually sat at around 7 or 8 g/dL. Now it’s almost 11 g/dL. I’m so happy about that. I want to pick up my camera again and focus more on the moments that make me, me.”
Data Behind the FDA Approval of FABHALTA
24-week study in adults with PNH who had received a C5 inhibitor:
FABHALTA was studied in a clinical trial of 97 participants who were previously treated with a complement (C5) inhibitor for at least 6 months. Out of the 97 participants, 62 adults switched to FABHALTA 200 mg twice a day and 35 adults remained on their C5 inhibitor (23 on SOLIRIS® and 12 on ULTOMIRIS®).1,4
- 82% of participants taking FABHALTA (51/62) vs 0% taking a C5 inhibitor (0/35) experienced an increase in hemoglobin level of ≥2 g/dL without the need for red blood cell (RBC) transfusions after 24 weeks4
- After 24 weeks,68% of people taking FABHALTA (42/62) achieved normalized hemoglobin levels of ≥12 g/dL without the need for RBC transfusions compared to 0% (0/35) taking a C5 inhibitor4
During this study, these adverse reactions were reported by >5% of adults with PNH:
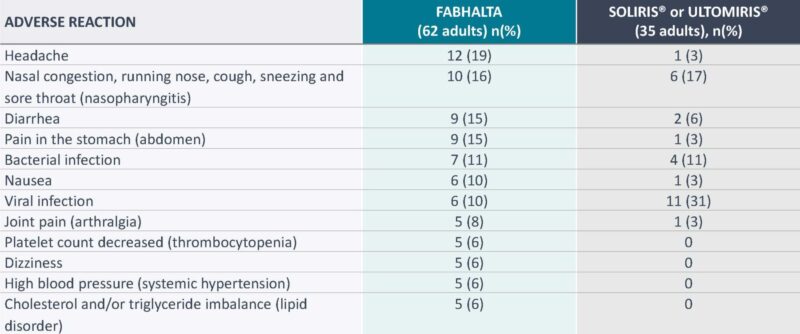
- Serious adverse reactions (kidney infection, urinary tract infection, and COVID-19) were reported in two people (3%) with PNH receiving FABHALTA1
- FABHALTA may increase your cholesterol and triglycerides, and your health care provider will do blood tests to check these periodically during treatment1
- Rash was reported in two people (3%) taking FABHALTA1
Because of the risk of serious infection caused by encapsulated bacteria, FABHALTA is only available through a Risk Evaluation and Mitigation Strategy (REMS) program that requires vaccinations.1 See Getting Started on FABHALTA (iptacopan) | PNH | HCP to learn more about the risk of serious infection and the need for vaccinations.
Rebuilding a Strong Foundation
Today, Garrett is back to enjoying the great outdoors and feels confident about his PNH care plan. He recently turned thirty, married Kait, and together they enjoy having a little boy at home and doing fun activities. He has even started to consider pursuing his hobby professionally. “Before being diagnosed, I had wanted to go to film school. I can now focus on building a strong foundation for myself and revisiting film, photography, art – and even tattoos! Everyone has a story that they want to tell, and if I can make an impact on someone by sharing mine, that would be amazing.”
Garrett also credits his open communication and collaboration with his care team as key to his progress as “medical advice from a trusted specialist means the world to me.”
“Don’t be afraid to ask questions,” he says. “From primary care to specialists, they’re well-equipped with answers or will find them.”
Recently, Garrett chose to share his experience as part of “The Open Road,” a video series featuring people living with PNH. For him, it was an opportunity to connect with others and offer encouragement to those who may be struggling. “Being able to make something good out of my experience is one of the best things I could ask for. I’ll always advocate for the person that doesn’t feel like they have a voice and fight for them. I also want others, especially those newly diagnosed, to know it’s going to be ok and that they’re not alone. A rare disease can make you feel alone and isolated, but there are treatments, support groups and community out there. You just have to take it one step at a time.”
Learn how he worked with his doctor to explore a treatment switch—and visit https://www.FABHALTA.com/pnh/resources to hear his story and more from others living with PNH.
Approved Use
What is FABHALTA?
FABHALTA is a prescription medicine used to treat adults with paroxysmal nocturnal hemoglobinuria (PNH).
It is not known if FABHALTA is safe and effective in children.
Important Safety Information
What is the most important information I should know about FABHALTA?
FABHALTA is a medicine that affects part of your immune system and may lower your ability to fight infections.
- FABHALTA increases your chance of getting serious infections caused by encapsulated bacteria, including Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b. These serious infections may quickly become life-threatening or fatal if not recognized and treated early.
- You must complete or update your vaccinations against Streptococcus pneumoniae and Neisseria meningitidis at least 2 weeks before your first dose of FABHALTA.
- If you have not completed your vaccinations and FABHALTA therapy must be started right away, you should receive the required vaccinations as soon as possible.
- If you have not been vaccinated and FABHALTA must be started right away, you should also receive antibiotics to take for as long as your health care provider tells you.
- If you have been vaccinated against these bacteria in the past, you might need additional vaccinations before starting FABHALTA. Your health care provider will decide if you need additional vaccinations.
- Vaccines do not prevent all infections caused by encapsulated bacteria. Call your health care provider or get emergency medical care right away if you have any of these signs and symptoms of a serious infection:
- Fever with or without shivers or chills
- Fever with chest pain and cough
- Fever with high heart rate
- Headache and fever
- Confusion
- Clammy skin
- Fever and rash
- Fever with breathlessness or fast breathing
- Headache with nausea or vomiting
- Headache with stiff neck or stiff back
- Body aches with flu-like symptoms
- Eyes sensitive to light
Your health care provider will give you a Patient Safety Card about the risk of serious infections. Carry it with you at all times during treatment and for 2 weeks after your last dose of FABHALTA. Your risk of serious infections may continue for a few weeks after your last dose of FABHALTA. It is important to show this card to any health care provider who treats you. This will help them diagnose and treat you quickly.
FABHALTA is only available through a program called the FABHALTA Risk Evaluation and Mitigation Strategy (REMS). Before you can take FABHALTA, your health care provider must:
- Enroll in the FABHALTA REMS program.
- Counsel you about the risk of serious infections caused by certain bacteria.
- Give you information about the symptoms of serious infections.
- Make sure that you are vaccinated against serious infections caused by encapsulated bacteria and that you receive antibiotics if you need to start FABHALTA right away and you are not up-to-date on your vaccinations.
- Give you a Patient Safety Card about your risk of serious infections.
Who should NOT take FABHALTA?
Do not take FABHALTA if you:
- Are allergic to FABHALTA or any of the ingredients in FABHALTA.
- Have a serious infection caused by encapsulated bacteria, including Streptococcus pneumoniae, Neisseria meningitidis, or Haemophilus influenzae type b, when you are starting FABHALTA.
Before you take FABHALTA, tell your health care provider about all your medical conditions, including if you:
- Have an infection or fever.
- Have liver problems.
- Are pregnant or plan to become pregnant. It is not known if FABHALTA will harm your unborn baby.
- Are breastfeeding or plan to breastfeed. It is not known if FABHALTA passes into your breast milk. You should not breastfeed during treatment and for 5 days after your final dose of FABHALTA.
Tell your health care provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking FABHALTA with certain other medicines may affect the way FABHALTA works and may cause side effects.
Know the medicines you take and the vaccines you receive. Keep a list of them to show your health care provider and pharmacist when you get a new medicine.
If you have PNH and you stop taking FABHALTA, your health care provider will need to monitor you closely for at least 2 weeks after stopping FABHALTA. Stopping FABHALTA may cause a breakdown of red blood cells due to PNH.
Symptoms or problems that can happen due to breakdown of red blood cells include:
- Decreased hemoglobin level in your blood
- Blood in your urine
- Shortness of breath
- Trouble swallowing
- Tiredness
- Pain in the stomach (abdomen)
- Blood clots, stroke, and heart attack
- Erectile dysfunction (ED)
It is important you take FABHALTA exactly as your health care provider tells you to lower the possibility of breakdown of red blood cells due to PNH.
What are the possible side effects of FABHALTA?
FABHALTA may cause serious side effects, including:
- See “What is the most important information I should know about FABHALTA?”
- Increased cholesterol and triglyceride (lipid) levels in your blood. Your health care provider will do blood tests to check your cholesterol and triglycerides during treatment with FABHALTA. Your health care provider may start you on a medicine to lower your cholesterol if needed.
The most common side effects of FABHALTA in adults include:
- Headache
- Nasal congestion, runny nose, cough, sneezing, and sore throat (nasopharyngitis)
- Diarrhea
- Pain in the stomach (abdomen)
- Infections (bacterial and viral)
- Nausea
- Rash
Tell your health care provider about any side effect that bothers you or that does not go away. These are not all the possible side effects of FABHALTA. Call your doctor for medical advice about side effects.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Please see full Prescribing Information, including Boxed WARNING and Medication Guide.
References:
-
- FABHALTA (iptacopan) Prescribing Information. Novartis Pharmaceuticals Corp.
- Cançado RD, Araújo A da S, Sandes AF, et al. Consensus statement for diagnosis and treatment of paroxysmal nocturnal haemoglobinuria.Hematol Transfus Cell Ther. 2021;43(3):341–348. doi:10.1016/j.htct.2020.06.006
- Dingli D, Matos JE, Lehrhaupt K, et al. The burden of illness in patients with paroxysmal nocturnal hemoglobinuria receiving treatment with the C5-inhibitors eculizumab or ravulizumab: results from a US patient survey. Ann Hematol. 2022;101(2):251-263. doi:10.1007/s00277-021-04715-5
- Peffault de Latour R, Rӧth A, Kulasekararaj A, et al. Oral Monotherapy with Iptacopan, a Proximal Complement Inhibitor of Factor B, Has Superior Efficacy to Intravenous Terminal Complement Inhibition with Standard of Care Eculizumab or Ravulizumab and Favorable Safety in Patients with Paroxysmal Nocturnal Hemoglobinuria and Residual Anemia: Results from the Randomized, Active-Comparator-Controlled, Open-Label, Multicenter, Phase III APPLY-PNH Study. Presented at: 64th American Society of Hematology Annual Meeting & Exposition (ASH); December 10-13, 2022; New Orleans, LA.
![]()
Novartis Pharmaceuticals Corporation





